With new thicker solid mu-metal magnetic shields (2 layers), the clock transition in our 88Sr+ experiments shows a transform-limited linewidth of ca 10 Hz using a 72ms probe pulse. More testing to follow, as we try to lengthen the probe-pulse and search for the ultimate linewidth limit.
Tag: strontium
Second run of 88Sr+ optical clock
A second run of the optical clock on 2020-06-11 - this is a 64x speedup of the overnight live-stream:
April Fools Ion
Despite the lockdown, some trapping in the lab this week. First single-ion experiments this year, after the 405 nm laser broke in January. Not much going on in these videos, but at least it's a single ion!
First clock-transition linewidth measurement give about a 200 Hz wide transition, so around 5e-13 fractionally.
clock-transition scan at 128x speedup
Recorded at 2x speed with OBS from the youtube live-stream, then converted to MP4 with VLC, then run twice through Garmin Virb Edit producing 8x speedup both times.
88Sr+ clock transition in a low magnetic field
We've completed a second magnetic shielding layer, based on the same plywood+METGLAS concept as the first shield. This should further shield the 88Sr+ ion from unwanted magnetic field fluctuations. To further reduce the DC-field we've now applied a counter-field using a few milliAmps of current through three coils that surround the ion trap.
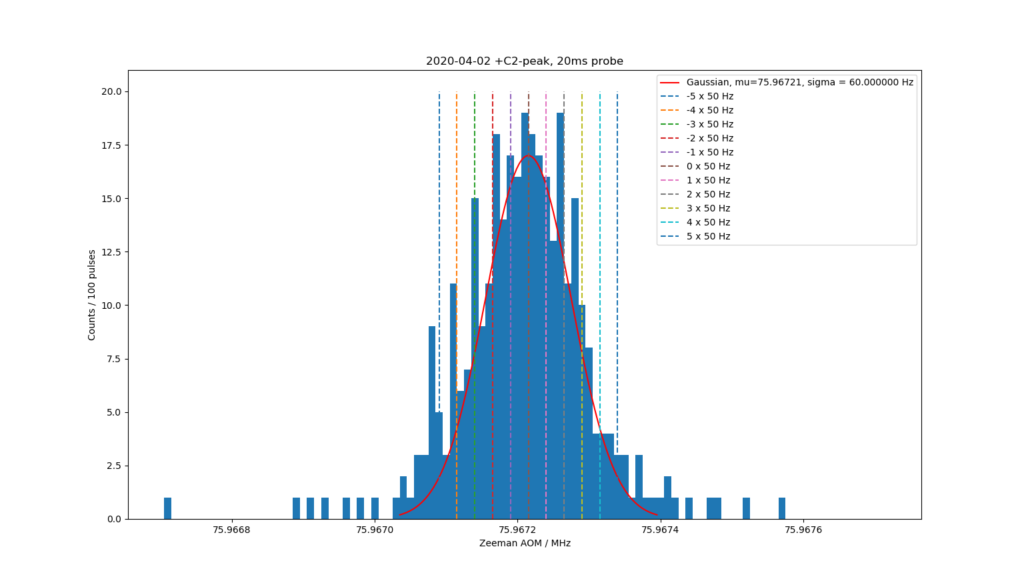
When the Zeeman components are this close together (the field is <0.4 uT) it is fairly quick to scan over the components. Here we see the four innermost pairs of peaks +/-C1 through +/-C4 of the clock-transition at 445 THz (674nm red light!). One scan runs in about one hour - and will be plotted on top of the older scans. We shoot 100 pulses of the laser-light at the ion and the height of the bar shows how many times we successfully drove the ion into the dark clock-state.
~800Hz wide clock-transition in 88Sr+
Here's the latest preliminary result from the VTT MIKES 88Sr+ ion clock (in case you missed the live-stream!):
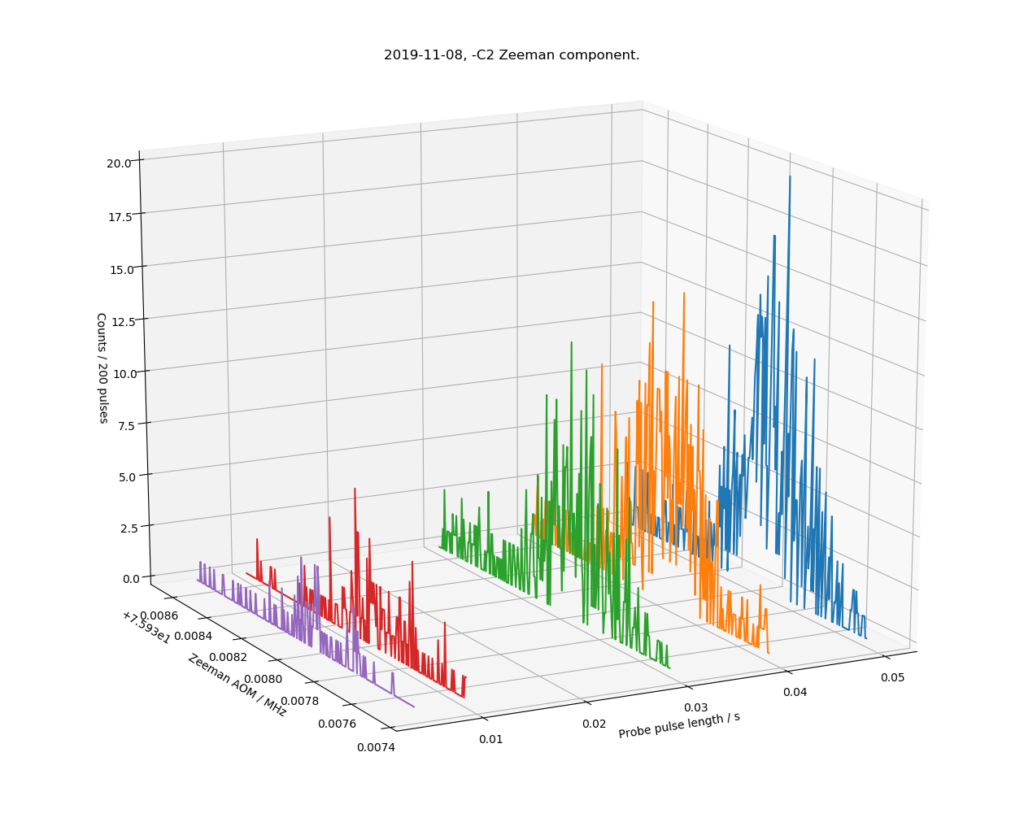
This shows a ~2 kHz slice (left axis, double the 1 kHz shown, because we plot the input frequency of a double-pass AOM) of the optical spectrum around 445 THz, where we expect to find the -C2 Zeeman component of the clock-transition in 88Sr+ (a secondary representation of the SI second). The right axis shows the probe-pulse length in seconds, where we see only a few percent excitation (z-axis) at short pulse lengths, but a clearer signal up to >10% at longer probe pulse lengths of 40 and 50 ms.
It ain't pretty, but considering the carrier is at 445 THz, this noisy and broad looking 800 Hz wide peak is still a measurement to a relative level of 2e-12. When fully operational a line-width of <10 Hz (2e-14) is expected.
Our experiment now has one metglas magnetic shield. This particular Zeeman component (-C2) has a sensitivity of 11 kHz/uT, so the observed linewidth of 800 Hz could be caused by a low-frequency AC magnetic field with an amplitude of 30-40 nT or so. We think the remaining DC-field is around 1.6 uT (down from 66 uT without the metglas shield).
Among other improvements, next is building a second metglas shield, to reduce AC fluctuations in the magnetic shield even further. Stay tuned...
Trapping again
Since about July 15th our single-ion experiment has been in maintenance-mode, but today again some trapping!
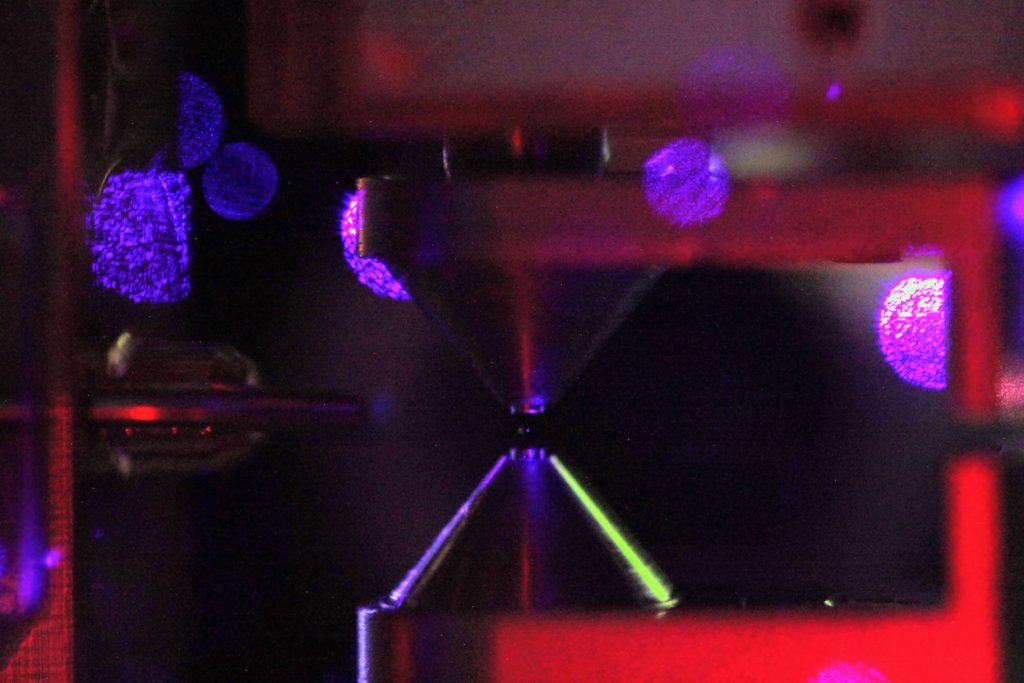
The central 'pale blue dot' is a single 88Sr+ ion trapped between the inner electrodes (cylindrical) which are driven with a ~400 Vpp ~16 MHz sine-wave to create a Paul trap. The outer conical electrodes are grounded. The window-reflections of the 422nm cooling laser create the larger circular spots in the top half of the picture. A single laser-cooled 88Sr+ ion emits around 10 million photons per second, making it easily visible on 1s or longer exposures with a DSLR. Sigma 105mm/F2.8 macro objective with a short (13mm?) extension-tube.
Live stream:
Lab live-stream: Trapped 88Sr+ ion
Tuesday Trapping
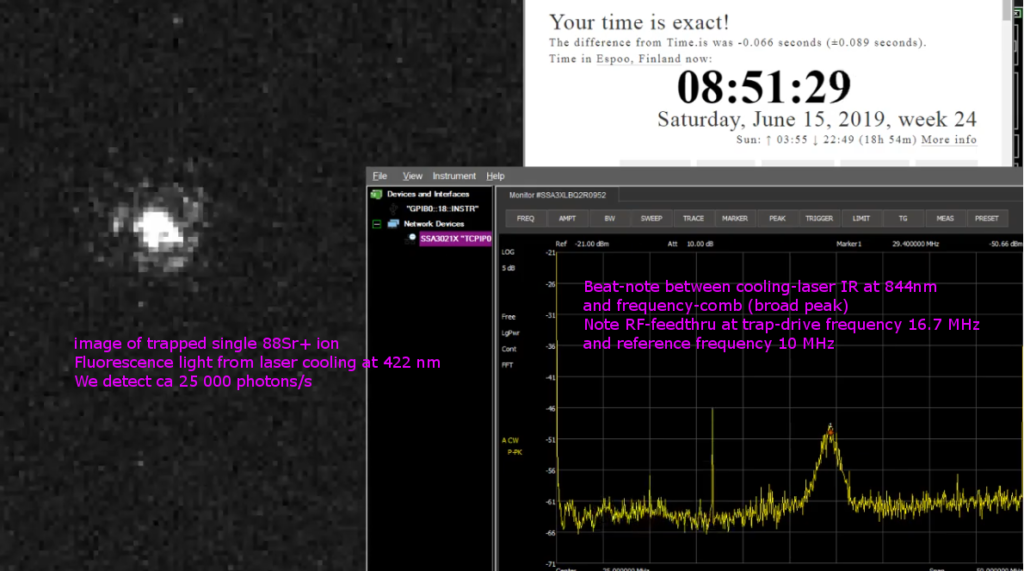
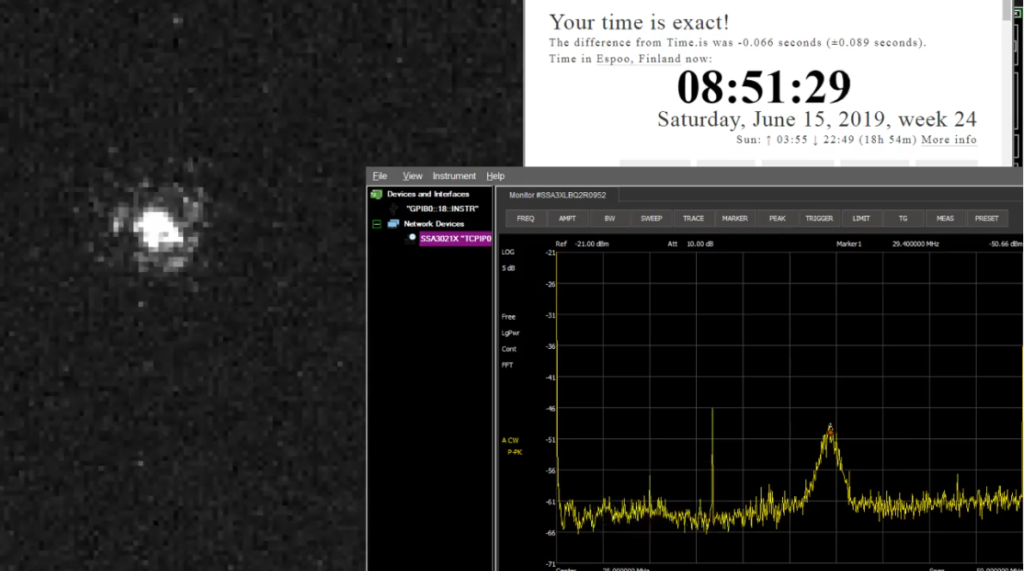
Strontium ion(s) trapped!
A major milestone towards the ion clock was reached today: we trapped the first Strontium ions!
This involves first heating a dispenser that contains Strontium atoms with about 4-5 W (4 A and 1 V). The atoms that fly out of the dispenser are then photoionized using two blue lasers, one at 461 nm and another at 405 nm (we use a laser from a blue-ray drive for this!). We now have Sr+ ions that can be trapped in a Paul trap. A high-voltage (300 Vpp) ~10 MHz sine-wave is applied to the electrodes of the trap. Another blue laser at 422 nm is then used both for laser-cooling and as a means to detect the ion. What we see in the video is the ion fluorescence at 422 nm when it jumps down to the ground state from an excited state. The excited state can also decay into a dark state and we need a re-pump laser at 1092 nm to keep the ion in the Doppler cooling cycle. The fluorescence emitted from one or a few ions is very weak, and we used an image-intensifier and a CCD camera with 500 ms to 1 s exposure time for this video.
The camera software produces FITS frames as output. I used these commands to make the video:
mogrify -format png *.fts # convert to PNG mogrify -crop "640x480+436+315" +repage *.png # crop to the interesting area mogrify -contrast-stretch 10x100 *.png # improve contrast ffmpeg -r 3 -f image2 -i 'myframes_%02d.png' -qscale 1 'video.avi'
On the last line "3" is the desired frame-rate of the output. I then concatenated a few of these videos together with
mencoder video1.avi video2.avi -mf fps=3 -oac copy -of lavf -ovc copy -lavcopts aglobal=1:vglobal=1:coder=0:vcodec=mpeg4:vbitrate=4500 -vf scale=1280:720 -o output2.mp4